-
- Autorefraktor / Keratometer
- Corneale modulare Systeme
- Farbtest & Farbtafeln
- Gläserkästen
- Handstücke
- Kontaktgläser Einweg und Mehrweg
- Kopfophthalmoskope
- Mesotest (Mesopisches Kontrastsehen)
- Messbrille / Probierbrille
- Nahsehtest
- Ophthalmometer
- Ophthalmoskopie direkt & indirekt
- Probiergläserkästen
- Refraktionsgläser
- Refraktionsprüfsätze / Brillenkästen
- Refraktionszubehör
- Refraktometer
- Scheitelbrechwertmesser
- Sehprüfgeräte
- Sehtafeln
- Sehzeichenprojektoren
- Spaltlampen & Zubehör
- Straßenverkehrsbezogener Sehtest
- Teststreifen
- Tonometer / Tonometrie
- Tonometerzubehör
-
- Abberometer und Wellenfrontabberometer
- Anomaloskop
- Elektrophysiologie
- Endothelmikroskope
- Funduskameras
- Hornhauttopographie
- IOL-Master
- Keratograph
- Keratometer
- Optische Kohärenztomographie (OCT)
- Pachymetrie
- Perimetrie und Zubehör
- Pupillometer
- Retina-Untersuchungsgeräte
- Retinometer
- Scheimpflugkameras
- Topographie (-Systeme)
- Ultraschall-Biometrie-Geräte
-
-
-
- Autoklaven & Sterilisationszubehör
- Chirurgielupen
- Crosslinking
- Crosslinking Zubehör
- Diamantmesser
- Glaukomchirurgie
- Hornhaut
- Hornhautkennzeichnung
- Hornhautstanzen
- Implantationsbesteck
- Injektoren
- Instrumente
- Instrumente (wiederverwendbar)
- Kataraktchirurgie
- Koagulationsgeräte
- Kryochirurgie-Geräte
- Lidsperrer
- Messer
- Mikrokeratome
- OP-Lampen
- OP-Liegen
- OP-Lüftungs- und Klimageräte
- OP-Mikroskope
- OP-Tische
- OP-Zubehör
- Operateurstühle
- Pendelmarkierer
- Phako-Zubehör
- Phakoemulsifikations-Geräte
- Pinzette
- Strabismusscheren
- Trepane
- Ultraschall-Reinigungsgeräte
- Vitrektomiemaschinen
- Vitrektomiezubehör
- Wetlab-.Zubehör
-
-
- Dienstleistungen
- Drehstühle & Drehhocker
- Ersatzlampen
- Fußschalter
- Gerätetische
- Glasschleifgeräte
- Karteischränke
- Lagerungshilfen
- Lupen Einweg und Mehrweg
- Patientenstühle
- Phoroptoren
- Refraktions- und Untersuchungseinheiten
- Refraktions- und Untersuchungsstühle
- Schreibtische
- Untersuchungseinheiten Umrüstsatz
- Untersuchungsgeräte
- Video-Betrachtungs und -Dokumentationseinrichtungen
-
- Anfärbelösungen
- Augenspülung
- Fluoreszenzangiographie
- Glaukom Implantate
- Hyaluronsäure
- Hygiene Produkte
- Implantate
- Instrumente (einmal)
- Intraokularlinsen
- Iris Implantate
- Irisdiaphragma
- Kapselspannringe
- Nahtmaterial
- OP-Abdeckung
- OP-Bedarf
- OP-Mantel
- OP-Sets
- Ophthalmologische Gase
- Punctum Plugs
- Retina-Implantat
- Silikonöl
- Spüllösungen (intraokulare)
- Verbandslinsen
- Viskoelastika
- IOL
- OCT
-
Allgemeine Augenuntersuchungen
- Autorefraktor / Keratometer
- Corneale modulare Systeme
- Farbtest & Farbtafeln
- Gläserkästen
- Handstücke
- Kontaktgläser Einweg und Mehrweg
- Kopfophthalmoskope
- Mesotest (Mesopisches Kontrastsehen)
- Messbrille / Probierbrille
- Nahsehtest
- Ophthalmometer
- Ophthalmoskopie direkt & indirekt
- Probiergläserkästen
- Refraktionsgläser
- Refraktionsprüfsätze / Brillenkästen
- Refraktionszubehör
- Refraktometer
- Scheitelbrechwertmesser
- Sehprüfgeräte
- Sehtafeln
- Sehzeichenprojektoren
- Spaltlampen & Zubehör
- Straßenverkehrsbezogener Sehtest
- Teststreifen
- Tonometer / Tonometrie
- Tonometerzubehör
-
Bildgebung und spezielle Augenuntersuchungen
- Abberometer und Wellenfrontabberometer
- Anomaloskop
- Elektrophysiologie
- Endothelmikroskope
- Funduskameras
- Hornhauttopographie
- IOL-Master
- Keratograph
- Keratometer
- Optische Kohärenztomographie (OCT)
- Pachymetrie
- Perimetrie und Zubehör
- Pupillometer
- Retina-Untersuchungsgeräte
- Retinometer
- Scheimpflugkameras
- Topographie (-Systeme)
- Ultraschall-Biometrie-Geräte
- Dienstleistungen und Beratung
- Heil- und Hilfsmittel, Kontaktlinsen
- Laser
-
OP-Ausstattung
- Autoklaven & Sterilisationszubehör
- Chirurgielupen
- Crosslinking
- Crosslinking Zubehör
- Diamantmesser
- Glaukomchirurgie
- Hornhaut
- Hornhautkennzeichnung
- Hornhautstanzen
- Implantationsbesteck
- Injektoren
- Instrumente
- Instrumente (wiederverwendbar)
- Kataraktchirurgie
- Koagulationsgeräte
- Kryochirurgie-Geräte
- Lidsperrer
- Messer
- Mikrokeratome
- OP-Lampen
- OP-Liegen
- OP-Lüftungs- und Klimageräte
- OP-Mikroskope
- OP-Tische
- OP-Zubehör
- Operateurstühle
- Pendelmarkierer
- Phako-Zubehör
- Phakoemulsifikations-Geräte
- Pinzette
- Strabismusscheren
- Trepane
- Ultraschall-Reinigungsgeräte
- Vitrektomiemaschinen
- Vitrektomiezubehör
- Wetlab-.Zubehör
- Orthoptik und Sehfunktionsprüfung
-
Praxisausstattung
- Dienstleistungen
- Drehstühle & Drehhocker
- Ersatzlampen
- Fußschalter
- Gerätetische
- Glasschleifgeräte
- Karteischränke
- Lagerungshilfen
- Lupen Einweg und Mehrweg
- Patientenstühle
- Phoroptoren
- Refraktions- und Untersuchungseinheiten
- Refraktions- und Untersuchungsstühle
- Schreibtische
- Untersuchungseinheiten Umrüstsatz
- Untersuchungsgeräte
- Video-Betrachtungs und -Dokumentationseinrichtungen
-
Verbrauchs- und OP-Material
- Anfärbelösungen
- Augenspülung
- Fluoreszenzangiographie
- Glaukom Implantate
- Hyaluronsäure
- Hygiene Produkte
- Implantate
- Instrumente (einmal)
- Intraokularlinsen
- Iris Implantate
- Irisdiaphragma
- Kapselspannringe
- Nahtmaterial
- OP-Abdeckung
- OP-Bedarf
- OP-Mantel
- OP-Sets
- Ophthalmologische Gase
- Punctum Plugs
- Retina-Implantat
- Silikonöl
- Spüllösungen (intraokulare)
- Verbandslinsen
- Viskoelastika
FCI SAS
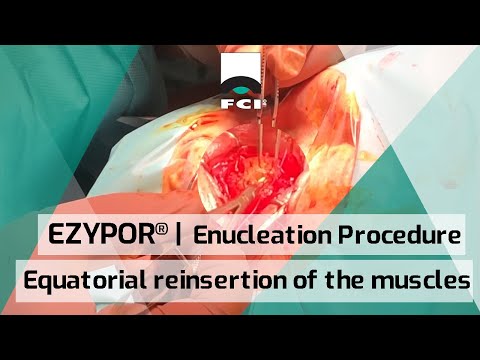
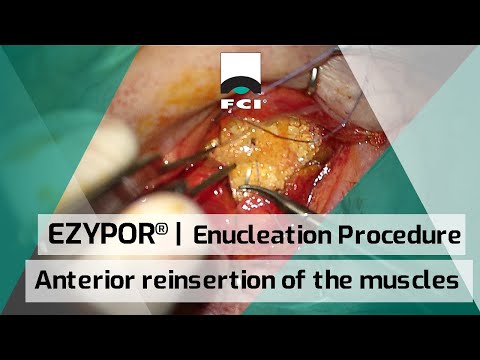
EZYPOR® | Ultra High Molecular Weight PolyEthylene Orbital Implant
EZYPOR® is FCI ultimate high density polyethylene orbital implant with a patented innovative suturing platform, indicated for enucleation, evisceration and secondary implantation.
Weitere ProduktinformationenEZYPOR® | Ultra High Molecular Weight PolyEthylene Orbital Implant
FCI EXCLUSIVE
EZYPOR® is FCI ultimate high density polyethylene orbital implant with a patented innovative suturing platform, indicated for enucleation, evisceration and secondary implantation.
EZYPOR® has a smooth anterior suturing surface with 8 suture tunnels allowing multiple suturing options.
Main characteristics:
- FCI Exclusive
- Made of Ultra High Molecular Weight PolyEthylene (UHMWPE)
- Porosity between 40% and 60% for optimum colonization of fibrovascular tissue
- Innovative and patented smooth anterior suturing platform
- 8 suture tunnels to facilitate needle insertion and muscles fixation
- No need for autologous graft
- No need for wrapping material
- Sterile
- EZYPOR® 12 mm and 14 mm do not have suturing platform
The EZYPOR® orbital implants are Class IIb medical devices manufactured by FCI SAS - Notified Body: GMED CE n ° 0459.