-
- Autorefraktor / Keratometer
- Corneale modulare Systeme
- Farbtest & Farbtafeln
- Gläserkästen
- Handstücke
- Kontaktgläser Einweg und Mehrweg
- Kopfophthalmoskope
- Mesotest (Mesopisches Kontrastsehen)
- Messbrille / Probierbrille
- Nahsehtest
- Ophthalmometer
- Ophthalmoskopie direkt & indirekt
- Probiergläserkästen
- Refraktionsgläser
- Refraktionsprüfsätze / Brillenkästen
- Refraktionszubehör
- Refraktometer
- Scheitelbrechwertmesser
- Sehprüfgeräte
- Sehtafeln
- Sehzeichenprojektoren
- Spaltlampen & Zubehör
- Straßenverkehrsbezogener Sehtest
- Teststreifen
- Tonometer / Tonometrie
- Tonometerzubehör
-
- Abberometer und Wellenfrontabberometer
- Anomaloskop
- Elektrophysiologie
- Endothelmikroskope
- Funduskameras
- Hornhauttopographie
- IOL-Master
- Keratograph
- Keratometer
- Optische Kohärenztomographie (OCT)
- Pachymetrie
- Perimetrie und Zubehör
- Pupillometer
- Retina-Untersuchungsgeräte
- Retinometer
- Scheimpflugkameras
- Topographie (-Systeme)
- Ultraschall-Biometrie-Geräte
-
-
-
- Autoklaven & Sterilisationszubehör
- Chirurgielupen
- Crosslinking
- Crosslinking Zubehör
- Diamantmesser
- Glaukomchirurgie
- Hornhaut
- Hornhautkennzeichnung
- Hornhautstanzen
- Implantationsbesteck
- Injektoren
- Instrumente
- Instrumente (wiederverwendbar)
- Kataraktchirurgie
- Koagulationsgeräte
- Kryochirurgie-Geräte
- Lidsperrer
- Messer
- Mikrokeratome
- OP-Lampen
- OP-Liegen
- OP-Lüftungs- und Klimageräte
- OP-Mikroskope
- OP-Tische
- OP-Zubehör
- Operateurstühle
- Pendelmarkierer
- Phako-Zubehör
- Phakoemulsifikations-Geräte
- Pinzette
- Strabismusscheren
- Trepane
- Ultraschall-Reinigungsgeräte
- Vitrektomiemaschinen
- Vitrektomiezubehör
- Wetlab-.Zubehör
-
-
- Dienstleistungen
- Drehstühle & Drehhocker
- Ersatzlampen
- Fußschalter
- Gerätetische
- Glasschleifgeräte
- Karteischränke
- Lagerungshilfen
- Lupen Einweg und Mehrweg
- Patientenstühle
- Phoroptoren
- Refraktions- und Untersuchungseinheiten
- Refraktions- und Untersuchungsstühle
- Schreibtische
- Untersuchungseinheiten Umrüstsatz
- Untersuchungsgeräte
- Video-Betrachtungs und -Dokumentationseinrichtungen
-
- Anfärbelösungen
- Augenspülung
- Fluoreszenzangiographie
- Glaukom Implantate
- Hyaluronsäure
- Hygiene Produkte
- Implantate
- Instrumente (einmal)
- Intraokularlinsen
- Iris Implantate
- Irisdiaphragma
- Kapselspannringe
- Nahtmaterial
- OP-Abdeckung
- OP-Bedarf
- OP-Mantel
- OP-Sets
- Ophthalmologische Gase
- Punctum Plugs
- Retina-Implantat
- Silikonöl
- Spüllösungen (intraokulare)
- Verbandslinsen
- Viskoelastika
- IOL
- OCT
-
Allgemeine Augenuntersuchungen
- Autorefraktor / Keratometer
- Corneale modulare Systeme
- Farbtest & Farbtafeln
- Gläserkästen
- Handstücke
- Kontaktgläser Einweg und Mehrweg
- Kopfophthalmoskope
- Mesotest (Mesopisches Kontrastsehen)
- Messbrille / Probierbrille
- Nahsehtest
- Ophthalmometer
- Ophthalmoskopie direkt & indirekt
- Probiergläserkästen
- Refraktionsgläser
- Refraktionsprüfsätze / Brillenkästen
- Refraktionszubehör
- Refraktometer
- Scheitelbrechwertmesser
- Sehprüfgeräte
- Sehtafeln
- Sehzeichenprojektoren
- Spaltlampen & Zubehör
- Straßenverkehrsbezogener Sehtest
- Teststreifen
- Tonometer / Tonometrie
- Tonometerzubehör
-
Bildgebung und spezielle Augenuntersuchungen
- Abberometer und Wellenfrontabberometer
- Anomaloskop
- Elektrophysiologie
- Endothelmikroskope
- Funduskameras
- Hornhauttopographie
- IOL-Master
- Keratograph
- Keratometer
- Optische Kohärenztomographie (OCT)
- Pachymetrie
- Perimetrie und Zubehör
- Pupillometer
- Retina-Untersuchungsgeräte
- Retinometer
- Scheimpflugkameras
- Topographie (-Systeme)
- Ultraschall-Biometrie-Geräte
- Dienstleistungen und Beratung
- Heil- und Hilfsmittel, Kontaktlinsen
- Laser
-
OP-Ausstattung
- Autoklaven & Sterilisationszubehör
- Chirurgielupen
- Crosslinking
- Crosslinking Zubehör
- Diamantmesser
- Glaukomchirurgie
- Hornhaut
- Hornhautkennzeichnung
- Hornhautstanzen
- Implantationsbesteck
- Injektoren
- Instrumente
- Instrumente (wiederverwendbar)
- Kataraktchirurgie
- Koagulationsgeräte
- Kryochirurgie-Geräte
- Lidsperrer
- Messer
- Mikrokeratome
- OP-Lampen
- OP-Liegen
- OP-Lüftungs- und Klimageräte
- OP-Mikroskope
- OP-Tische
- OP-Zubehör
- Operateurstühle
- Pendelmarkierer
- Phako-Zubehör
- Phakoemulsifikations-Geräte
- Pinzette
- Strabismusscheren
- Trepane
- Ultraschall-Reinigungsgeräte
- Vitrektomiemaschinen
- Vitrektomiezubehör
- Wetlab-.Zubehör
- Orthoptik und Sehfunktionsprüfung
-
Praxisausstattung
- Dienstleistungen
- Drehstühle & Drehhocker
- Ersatzlampen
- Fußschalter
- Gerätetische
- Glasschleifgeräte
- Karteischränke
- Lagerungshilfen
- Lupen Einweg und Mehrweg
- Patientenstühle
- Phoroptoren
- Refraktions- und Untersuchungseinheiten
- Refraktions- und Untersuchungsstühle
- Schreibtische
- Untersuchungseinheiten Umrüstsatz
- Untersuchungsgeräte
- Video-Betrachtungs und -Dokumentationseinrichtungen
-
Verbrauchs- und OP-Material
- Anfärbelösungen
- Augenspülung
- Fluoreszenzangiographie
- Glaukom Implantate
- Hyaluronsäure
- Hygiene Produkte
- Implantate
- Instrumente (einmal)
- Intraokularlinsen
- Iris Implantate
- Irisdiaphragma
- Kapselspannringe
- Nahtmaterial
- OP-Abdeckung
- OP-Bedarf
- OP-Mantel
- OP-Sets
- Ophthalmologische Gase
- Punctum Plugs
- Retina-Implantat
- Silikonöl
- Spüllösungen (intraokulare)
- Verbandslinsen
- Viskoelastika
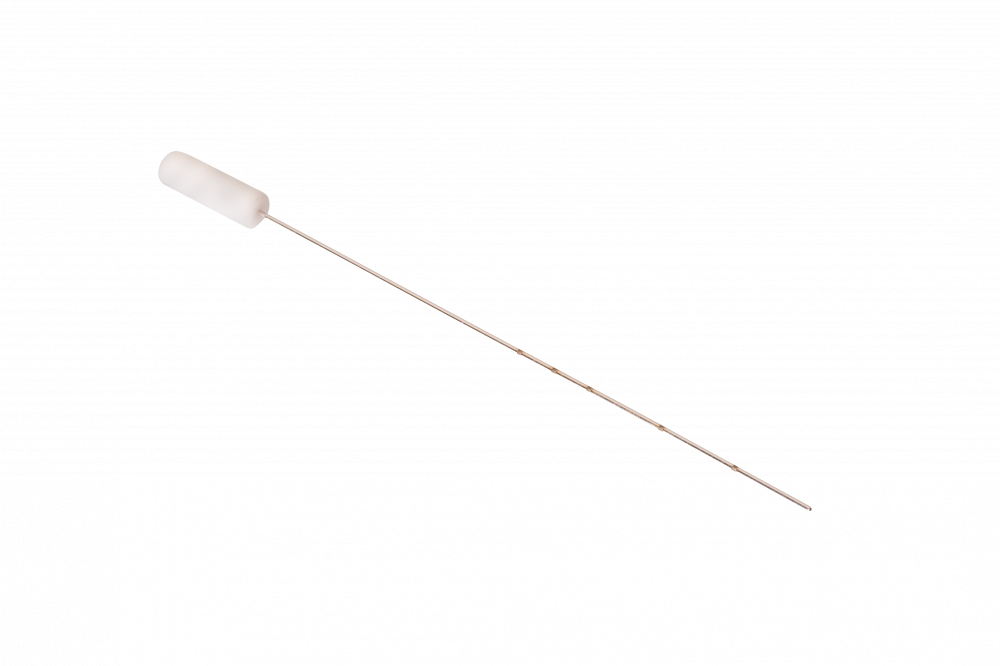
Masterka®
Masterka® is FCI latest innovation for treatment of congenital nasolacrimal duct obstruction resistant to probing.
Weitere ProduktinformationenMasterka®
FCI EXCLUSIVE
Masterka® is FCI latest innovation for treatment of congenital nasolacrimal duct obstruction resistant to probing.
Unlike the traditional “pulled” technique in which the stent is advanced through the nasolacrimal system and retrieved through the nose by pulling on the guide probe or thread, the Masterka® has no metallic probe or suture attached to it and, therefore, it is not pulled out of the nose.
Instead, the Masterka® is pushed into the nasolacrimal duct and anchored in place at the punctum by a plug-like fixation head.
Main characteristics:
- Easy to insert
- No nasal retrieval, no knot or sutures needed
- Less traumatic
- Available in 3 lenghts
- 3 mm plug collarette
- Requires sizer (S1.1289) to select the length & 0.3 mm disposable punctum dilator and plug inserter (S1.3090)
- Sterile, Single use
Masterka® is a Class IIb medical devices manufactured by FCI S.A.S. - Notified Body: GMED CE n°0459.