-
- Autorefraktor / Keratometer
- Corneale modulare Systeme
- Farbtest & Farbtafeln
- Gläserkästen
- Handstücke
- Kontaktgläser Einweg und Mehrweg
- Kopfophthalmoskope
- Mesotest (Mesopisches Kontrastsehen)
- Messbrille / Probierbrille
- Nahsehtest
- Ophthalmometer
- Ophthalmoskopie direkt & indirekt
- Probiergläserkästen
- Refraktionsgläser
- Refraktionsprüfsätze / Brillenkästen
- Refraktionszubehör
- Refraktometer
- Scheitelbrechwertmesser
- Sehprüfgeräte
- Sehtafeln
- Sehzeichenprojektoren
- Spaltlampen & Zubehör
- Straßenverkehrsbezogener Sehtest
- Teststreifen
- Tonometer / Tonometrie
- Tonometerzubehör
-
- Abberometer und Wellenfrontabberometer
- Anomaloskop
- Elektrophysiologie
- Endothelmikroskope
- Funduskameras
- Hornhauttopographie
- IOL-Master
- Keratograph
- Keratometer
- Optische Kohärenztomographie (OCT)
- Pachymetrie
- Perimetrie und Zubehör
- Pupillometer
- Retina-Untersuchungsgeräte
- Retinometer
- Scheimpflugkameras
- Topographie (-Systeme)
- Ultraschall-Biometrie-Geräte
-
-
-
- Autoklaven & Sterilisationszubehör
- Chirurgielupen
- Crosslinking
- Crosslinking Zubehör
- Diamantmesser
- Glaukomchirurgie
- Hornhaut
- Hornhautkennzeichnung
- Hornhautstanzen
- Implantationsbesteck
- Injektoren
- Instrumente
- Instrumente (wiederverwendbar)
- Kataraktchirurgie
- Koagulationsgeräte
- Kryochirurgie-Geräte
- Lidsperrer
- Messer
- Mikrokeratome
- OP-Lampen
- OP-Liegen
- OP-Lüftungs- und Klimageräte
- OP-Mikroskope
- OP-Tische
- OP-Zubehör
- Operateurstühle
- Pendelmarkierer
- Phako-Zubehör
- Phakoemulsifikations-Geräte
- Pinzette
- Strabismusscheren
- Trepane
- Ultraschall-Reinigungsgeräte
- Vitrektomiemaschinen
- Vitrektomiezubehör
- Wetlab-.Zubehör
-
-
- Dienstleistungen
- Drehstühle & Drehhocker
- Ersatzlampen
- Fußschalter
- Gerätetische
- Glasschleifgeräte
- Karteischränke
- Lagerungshilfen
- Lupen Einweg und Mehrweg
- Patientenstühle
- Phoroptoren
- Refraktions- und Untersuchungseinheiten
- Refraktions- und Untersuchungsstühle
- Schreibtische
- Untersuchungseinheiten Umrüstsatz
- Untersuchungsgeräte
- Video-Betrachtungs und -Dokumentationseinrichtungen
-
- Anfärbelösungen
- Augenspülung
- Fluoreszenzangiographie
- Glaukom Implantate
- Hyaluronsäure
- Hygiene Produkte
- Implantate
- Instrumente (einmal)
- Intraokularlinsen
- Iris Implantate
- Irisdiaphragma
- Kapselspannringe
- Nahtmaterial
- OP-Abdeckung
- OP-Bedarf
- OP-Mantel
- OP-Sets
- Ophthalmologische Gase
- Punctum Plugs
- Retina-Implantat
- Silikonöl
- Spüllösungen (intraokulare)
- Verbandslinsen
- Viskoelastika
- IOL
- OCT
-
Allgemeine Augenuntersuchungen
- Autorefraktor / Keratometer
- Corneale modulare Systeme
- Farbtest & Farbtafeln
- Gläserkästen
- Handstücke
- Kontaktgläser Einweg und Mehrweg
- Kopfophthalmoskope
- Mesotest (Mesopisches Kontrastsehen)
- Messbrille / Probierbrille
- Nahsehtest
- Ophthalmometer
- Ophthalmoskopie direkt & indirekt
- Probiergläserkästen
- Refraktionsgläser
- Refraktionsprüfsätze / Brillenkästen
- Refraktionszubehör
- Refraktometer
- Scheitelbrechwertmesser
- Sehprüfgeräte
- Sehtafeln
- Sehzeichenprojektoren
- Spaltlampen & Zubehör
- Straßenverkehrsbezogener Sehtest
- Teststreifen
- Tonometer / Tonometrie
- Tonometerzubehör
-
Bildgebung und spezielle Augenuntersuchungen
- Abberometer und Wellenfrontabberometer
- Anomaloskop
- Elektrophysiologie
- Endothelmikroskope
- Funduskameras
- Hornhauttopographie
- IOL-Master
- Keratograph
- Keratometer
- Optische Kohärenztomographie (OCT)
- Pachymetrie
- Perimetrie und Zubehör
- Pupillometer
- Retina-Untersuchungsgeräte
- Retinometer
- Scheimpflugkameras
- Topographie (-Systeme)
- Ultraschall-Biometrie-Geräte
- Dienstleistungen und Beratung
- Heil- und Hilfsmittel, Kontaktlinsen
- Laser
-
OP-Ausstattung
- Autoklaven & Sterilisationszubehör
- Chirurgielupen
- Crosslinking
- Crosslinking Zubehör
- Diamantmesser
- Glaukomchirurgie
- Hornhaut
- Hornhautkennzeichnung
- Hornhautstanzen
- Implantationsbesteck
- Injektoren
- Instrumente
- Instrumente (wiederverwendbar)
- Kataraktchirurgie
- Koagulationsgeräte
- Kryochirurgie-Geräte
- Lidsperrer
- Messer
- Mikrokeratome
- OP-Lampen
- OP-Liegen
- OP-Lüftungs- und Klimageräte
- OP-Mikroskope
- OP-Tische
- OP-Zubehör
- Operateurstühle
- Pendelmarkierer
- Phako-Zubehör
- Phakoemulsifikations-Geräte
- Pinzette
- Strabismusscheren
- Trepane
- Ultraschall-Reinigungsgeräte
- Vitrektomiemaschinen
- Vitrektomiezubehör
- Wetlab-.Zubehör
- Orthoptik und Sehfunktionsprüfung
-
Praxisausstattung
- Dienstleistungen
- Drehstühle & Drehhocker
- Ersatzlampen
- Fußschalter
- Gerätetische
- Glasschleifgeräte
- Karteischränke
- Lagerungshilfen
- Lupen Einweg und Mehrweg
- Patientenstühle
- Phoroptoren
- Refraktions- und Untersuchungseinheiten
- Refraktions- und Untersuchungsstühle
- Schreibtische
- Untersuchungseinheiten Umrüstsatz
- Untersuchungsgeräte
- Video-Betrachtungs und -Dokumentationseinrichtungen
-
Verbrauchs- und OP-Material
- Anfärbelösungen
- Augenspülung
- Fluoreszenzangiographie
- Glaukom Implantate
- Hyaluronsäure
- Hygiene Produkte
- Implantate
- Instrumente (einmal)
- Intraokularlinsen
- Iris Implantate
- Irisdiaphragma
- Kapselspannringe
- Nahtmaterial
- OP-Abdeckung
- OP-Bedarf
- OP-Mantel
- OP-Sets
- Ophthalmologische Gase
- Punctum Plugs
- Retina-Implantat
- Silikonöl
- Spüllösungen (intraokulare)
- Verbandslinsen
- Viskoelastika
Ritleng®+
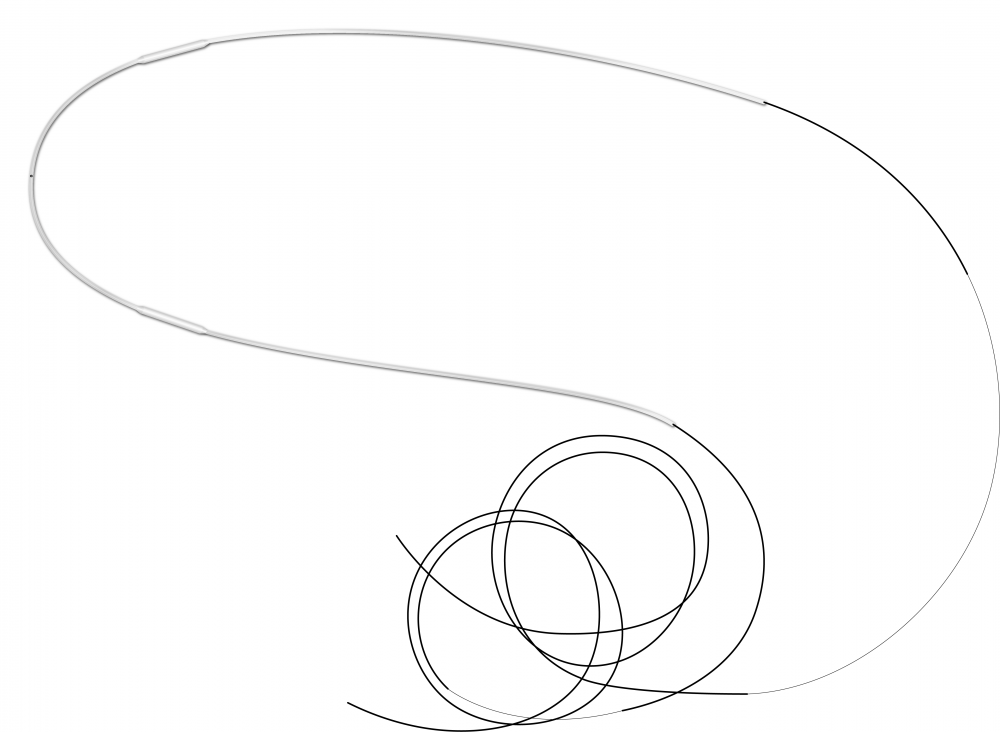
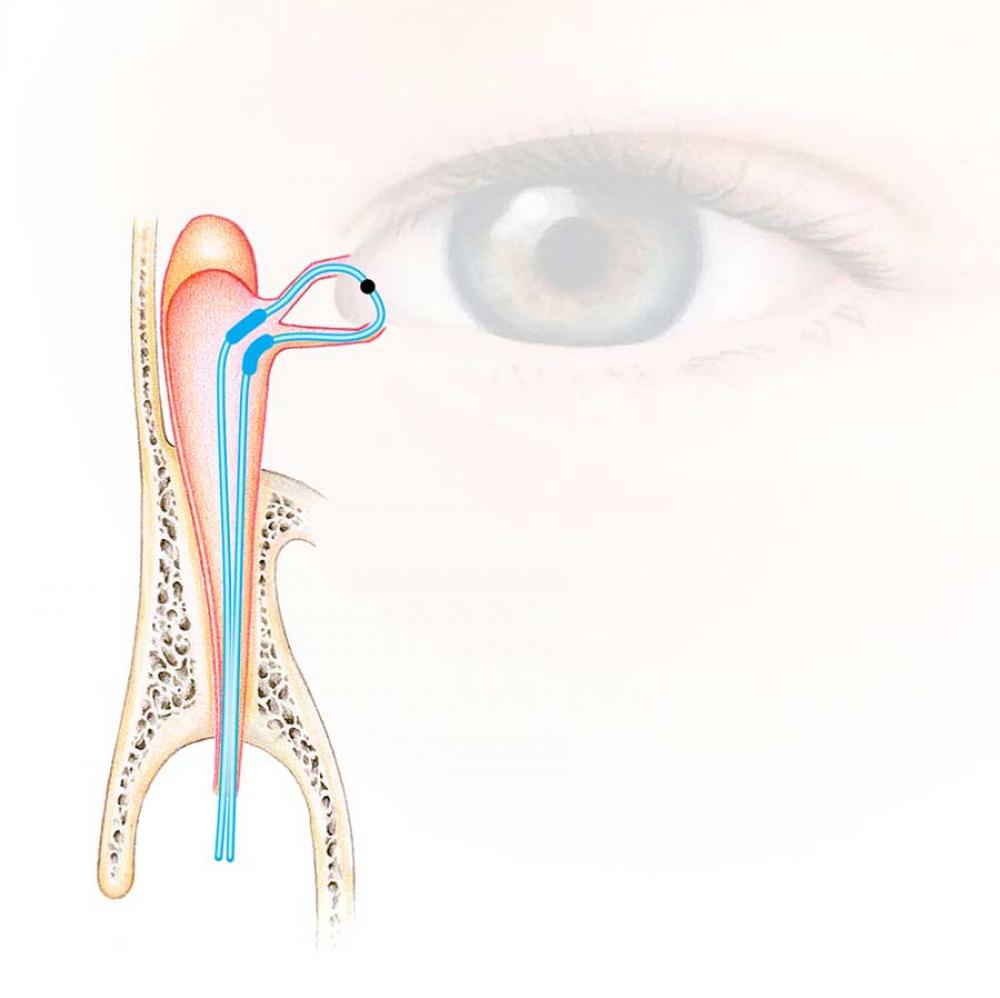
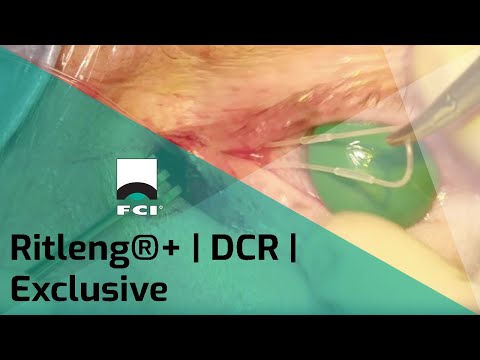
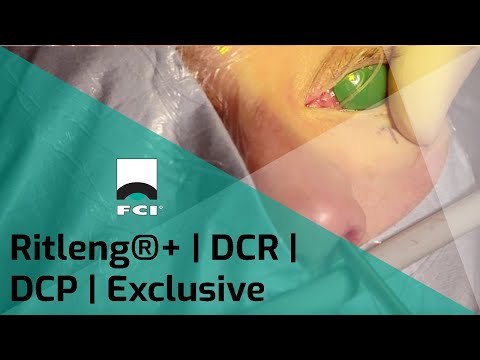
Ritleng®+ is FCI latest autostable bicanalicular nasolacrimal intubation indicated for epiphora, canalicular pathologies, congenital lacrimal duct obstruction and dacryocystorhinostomy (DCR).
Weitere ProduktinformationenRitleng®+
FCI EXCLUSIVE
Ritleng®+ is FCI latest autostable bicanalicular nasolacrimal intubation indicated for epiphora, canalicular pathologies, congenital lacrimal duct obstruction and dacryocystorhinostomy (DCR).
It consists of a silicone tube connected at each extremity to a PEEK thread guide. It is designed to reduce operating time and trauma for the patients, either in the intubation phase as well as for the removal of the device.
The intubation is self-retaining thanks to two wider silicone portions on the silicone tube. The surgical procedure is similar to the conventional Ritleng® lacrimal intubation and involves the use of Ritleng® instruments.
Main characteristics:
- FCI exclusive design
- Regular 0.64mm silicone tube for a comfortable fit in the intrapalpebral loop
- Wider 0.94mm silicone segments to be positioned in the lacrimal sac ensuring the relf-retaining feature of the intubation: no need to make knots in the nasal fossa
- Black mark to control the central placement and correct positioning of the silicone tube between the two punctums
- Easy removal from the nose
- Require Ritleng® instruments (S1.1460, S1.1470, S1.1480)
The Ritleng®+ intubation system is a Class IIb medical device manufactured by FCI S.A.S. - Notified Body: GMED CE n°0459.