Nanoscope’s Gene Therapy for Inherited Retinal Diseases Granted Sakigake and Orphan Drug Status in Japan
The most advanced optogenetic platform therapy in development worldwide: MCO-010 has received multiple accelerated regulatory designations across Japan, the United States and Europe, positioning the gene-agnostic intravitreal therapy at the forefront of clinical development for inherited retinal diseases with severe vision loss.
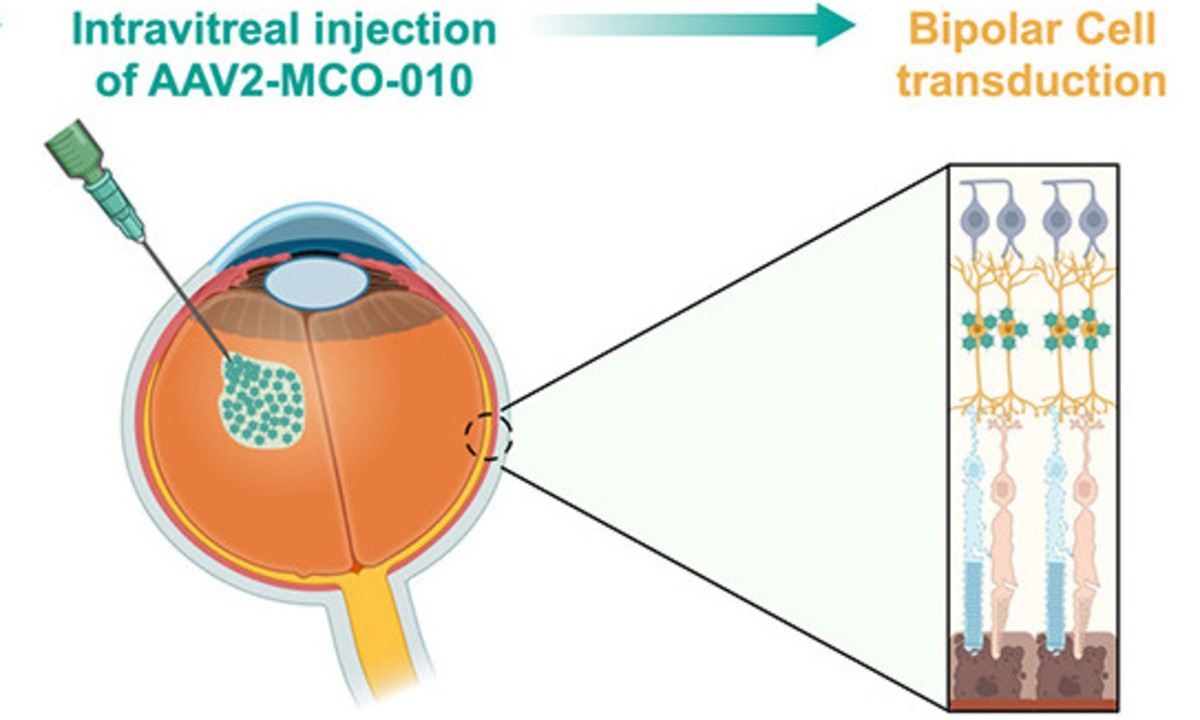
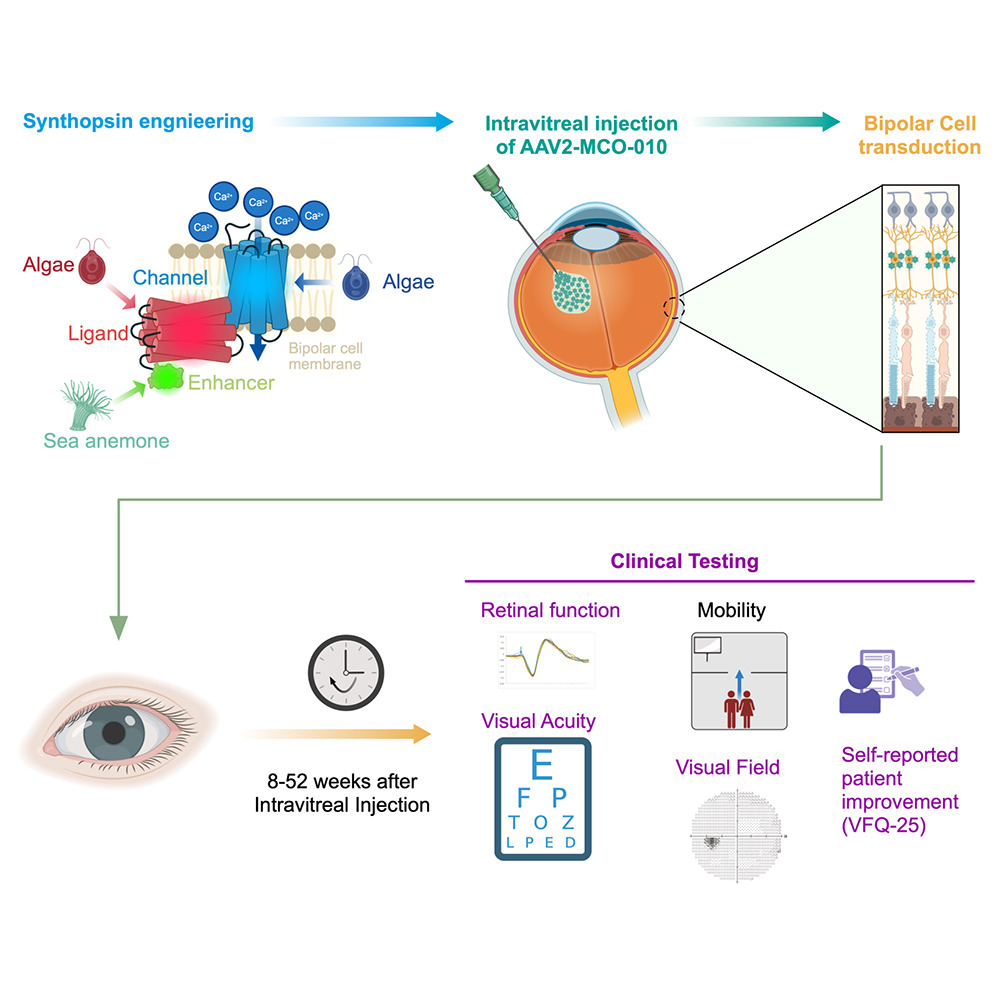
Japan’s Ministry of Health, Labour and Welfare (MHLW) has granted Sakigake and Orphan Drug designations to MCO-010, an optogenetic gene therapy under development for patients with severe vision loss due to inherited retinal diseases (IRDs). According to the developer, this is the first retinal gene therapy to receive both designations in Japan.
The Sakigake program is Japan’s accelerated regulatory pathway for innovative therapies addressing serious unmet medical needs and provides prioritized regulatory consultation and review by the Pharmaceuticals and Medical Devices Agency (PMDA). Orphan Drug designation applies to treatments for rare diseases with limited therapeutic options.
The decision in Japan adds to existing regulatory support in other major markets.
European Union (EMA)
Five Orphan designations spanning non-syndromic and syndromic rod- and cone-dominant dystrophies, as well as macular dystrophies
United States (FDA)
Orphan Drug and Fast Track designations for retinitis pigmentosa (RP)
Orphan Drug, Fast Track, and Regenerative Medicine Advanced Therapy (RMAT) designations for Stargardt disease (SD)
The proposed brand name MOGENRY™ has been conditionally accepted by FDA, EMA and PMDA.
Most advanced optogenetic platform therapy in development worldwide
With these regulatory pathways now established in Japan, the United States, and Europe, MCO-010 represents the most advanced optogenetic platform therapy in development worldwide. These coordinated designations support accelerated development timelines, enhanced regulatory engagement, and the opportunity to be the first optogenetic therapy approved across major markets.
About the MCO Platform
MCO is a one-time, in-office, intravitreal disease-agnostic therapy platform designed to restore vision in patients with photoreceptor degeneration, including Retinitis Pigmentosa (RP), Stargardt disease (SD), and geographic atrophy (GA). By activating highly dense bipolar retinal cells to become light sensitive, MCO utilizes the remaining visual circuitry following photoreceptor death. MCO treatment does not require genetic testing, invasive surgery, or repeat dosing, enabling broad patient applicability within existing retina office workflows.
A rolling Biologics License Application (BLA) for retinitis pigmentosa is currently under review by the U.S. Food and Drug Administration. Additional clinical development programs include Stargardt disease and geographic atrophy.
Source: Nanoscope