Stargardt Disease: Gene therapy receives FDA Orphan Drug Designation and UK CTA approval
AAVantgarde Bio has achieved two key regulatory milestones for its lead gene therapy program, AAVB-039, in development for treating Stargardt disease. The Italy-based company announced that it was granted orphan drug designation by FDA and received approval from the UK Medicines and Healthcare products Regulatory Agency (MHRA) for its clinical trial authorization (CTA) application.
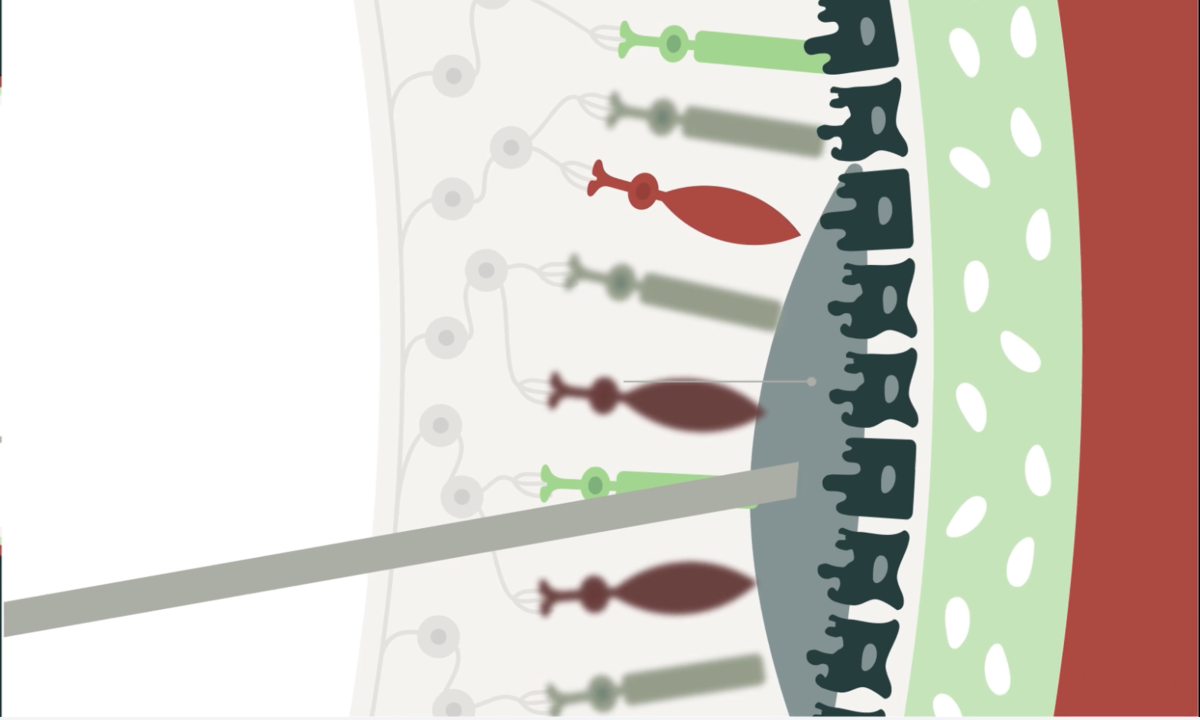
AAVB-039 addresses the root cause of the disease, benefitting patients with any ABCA4 mutation. It is currently being assessed in the CELESTE interventional clinical trial.
AAVantgarde Bio, a clinical-stage biotechnology company developing gene therapies for inherited retinal diseases, announced that the U.S. Food and Drug Administration (FDA) has granted AAVB-039 Orphan Drug Designation (ODD). AAVB-039 is the company’s investigational gene therapy for the treatment of Stargardt disease secondary to biallelic mutation in ABCA4. The company has also received Clinical Trial Authorisation (CTA) approval from the UK's Medicines and Healthcare products Regulatory Agency (MHRA).
Most common inherited form of macular degeneration
Stargardt disease is the most common inherited form of macular degeneration and is a leading cause of vision loss in children and young adults. AAVB-039 addresses the root cause of the disease by delivering the full-length ABCA4 protein, with the potential to benefit all patients with ABCA4 mutations.
The Investigational New Drug (IND) and CTA applications for AAVB-039 have been cleared to proceed by the FDA and the MHRA, respectively, and the program also holds Fast Track Designation in the US.
Platform enables delivery of large genes
AAVB-039 utilizes AAVantgarde’s proprietary dual AAV intein platform, which enables delivery of large genes to tissue and cells in vivo. The ABCA4 gene is 6.8 kilobases in length, too large to be packaged within a standard, single AAV vector.
The FDA’s Orphan Drug Designation program is intended to advance the development of drugs and biologics for rare diseases affecting fewer than 200,000 people in the United States. Benefits of ODD include tax credits for qualified clinical testing, waiving of certain FDA application fees, and, if approved, seven years of U.S. market exclusivity.
AAVB-039 is currently being evaluated in the Phase 1/2 CELESTE clinical trial, which assesses safety, tolerability, and preliminary efficacy of AAVB-039 in patients with Stargardt disease across three dose levels.
About AAVantgarde Bio
AAVantgarde Bio is a clinical stage, biotechnology company advancing best-in-class therapies for patients with inherited retinal diseases (IRDs). The company’s lead programs target Stargardt disease and retinitis pigmentosa due to Usher syndrome type 1B, two severe IRDs with no approved treatments. AAVB-039 and AAVB-081 are investigational, dual AAV gene therapies designed to address the root genetic causes of these diseases.
AAVantgarde is a spin-off of Telethon Institute for Genetics and Medicine (TIGEM), an international research institute based in Naples that is owned and managed by the Telethon Foundation, and of the University of Naples "Federico II".
Founded by Professor Alberto Auricchio
AAVantgarde was founded by Professor Alberto Auricchio, a pioneer in the field of gene therapy. As well as being Founder and CSO of AAVantgarde, Professor Auricchio is TIGEM Scientific Director and Professor of Medical Genetics at University “Federico II” in Naples, Italy. In 2024 he has been appionted as the new President of the European Society of Gene and Cell Therapy (ESGCT). This celebrates his long-standing contributions to gene therapy, particularly in treating genetic ocular and metabolic diseases. Auricchio has significantly advanced AAV vector technology. His research has led to important developments in treating inherited retinal diseases, notably contributing to Luxturna, the first FDA-approved gene therapy for an ocular condition.
Source: AAVantgarde Bio