Cancer Drug Shows Promise as Novel Treatment for Neovascular Eye Diseases
Singapore’s Health Sciences Authority (HSA) approves human trials for cancer drug PRL3-zumab showing promise as intravenous alternative to intravitreal injections in treating wet AMD and diabetic retinopathy.
Researchers have discovered that a cancer drug, originally developed at A*STAR Institute of Molecular and Cell Biology (A*STAR IMCB) in Singapore, shows potential as a new treatment approach for neovascular eye diseases.
In pre-clinical studies published in Nature Communications, PRL3-zumab effectively reduced leakage from damaged blood vessels showing promise as a potential new treatment for patients whose conditions do not respond well to current therapies.
The findings, from scientists at A*STAR IMCB and local biotech firm Intra-ImmuSG (IISG), point to a new option for treating wet age-related macular degeneration (AMD) and diabetic retinopathy, two of the most common causes of vision loss globally.
Addressing Limitations of Current Treatments
Currently, patients with these diseases require monthly intravitreal injections—a procedure that carries risks of infection and lens damage. Additionally, up to 45% of patients do not respond adequately to these treatments, highlighting the need for alternative approaches.
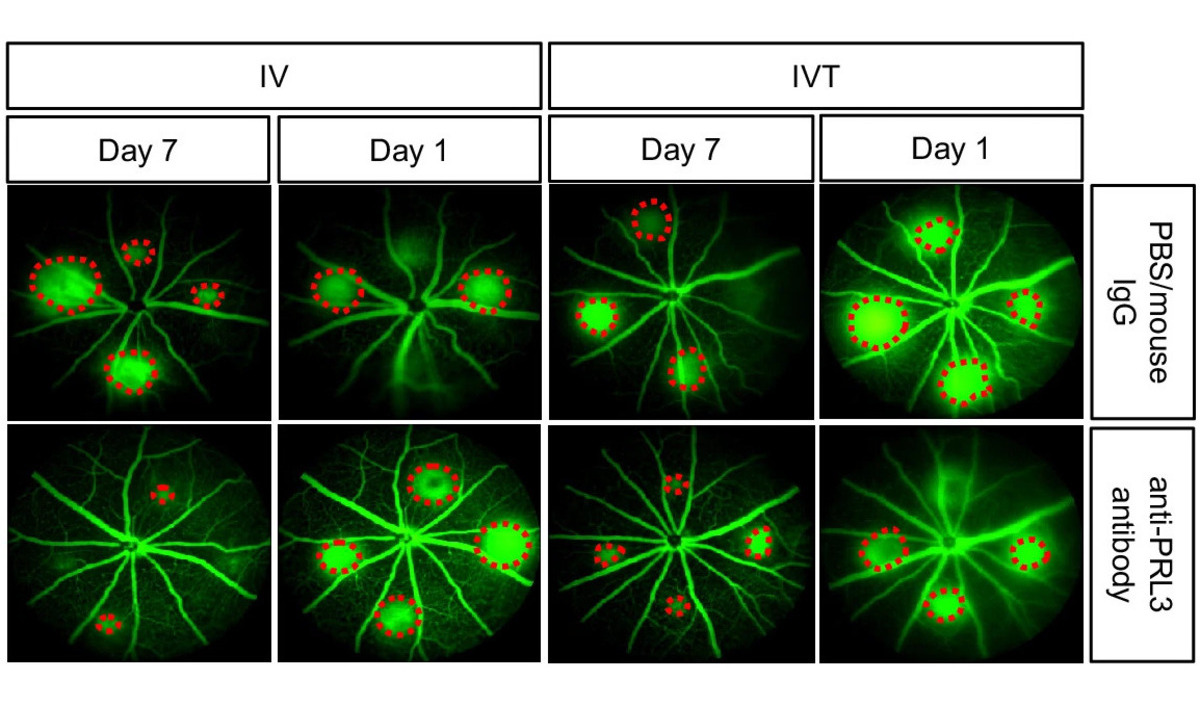
Reduction in abnormal blood vessel leakage
PRL3-zumab offers a different approach. Unlike current therapies, it can be administered intravenously. In pre-clinical studies, intravenous delivery showed an 86% greater reduction in abnormal blood vessel leakage compared to intravitreal injections, potentially preventing vision loss associated with these diseases.
The study findings indicate that intravenous delivery achieved more sustained therapeutic levels in the eye compared to traditional eye injections, while potentially avoiding injection-related complications.
The research team is now preparing for human trials following approval from Singapore’s Health Sciences Authority (HSA), granted on 16 June 2025. Clinical trials are expected to begin by late 2025, marking an important milestone in evaluating PRL3-zumab's potential as an ophthalmology treatment in Singapore.
PRL3-zumab has already completed Phase II trials in cancer patients with a favourable safety profile—data that provides useful background as researchers explore its application for eye diseases.
From Cancer Research to Ophthalmology Applications
The journey of PRL3-zumab illustrates how insights from cancer research can lead to breakthroughs in other medical fields. Professor Qi Zeng, Senior Principal Scientist at A*STAR IMCB and founder of IISG, initially identified the PRL3 protein in 1998 as a key factor in cancer metastasis. PRL3 (Phosphatase of Regenerating Liver 3) is a cancer-associated phosphatase that is overexpressed in a wide repertoire of cancer types. Subsequent studies revealed PRL3’s surprising role in eye diseases, opening a new frontier for treatment.
In their study the scientists report the specific upregulation of endogenous PRL3 protein in diseased choroid-RPE in choroidal neovascularization (CNV) mouse model and diseased retina in oxygen-induced retinopathy (OIR) mouse model, indicating PRL3’s role in neovascularization. Intravenous (IV) delivery of anti-PRL3 antibody in CNV model demonstrates superior efficacy in reducing vascular leakage compared to intravitreal (IVT) route due to larger dose permitted by IV. PRL3’s involvement in ocular pathological angiogenesis suggests the potential of repurposing PRL3-zumab to treat neovascular eye diseases.
“When I first identified PRL3 over two decades ago, I never imagined that our cancer research could also provide hope for patients facing blindness,” said Professor Qi Zeng, senior author on the study. “Seeing PRL3-zumab now potentially transform treatment for devastating eye conditions shows how fundamental scientific discoveries can lead to life-changing outcomes.”
"The repurposing of PRL3-zumab offers the possibility of a faster, more cost-effective and potentially safer path to developing treatments for these eye diseases," said Associate Professor Xinyi Su, Executive Director of A*STAR IMCB, also a co-author on the study. "This is made possible through the close collaborations in Singapore between our scientists, clinicians and clinician scientists."
Study citation: Ang, K.H., Thura, M., Tan, Q.S.W. et al. PRL3-zumab as an anti-angiogenic therapy in neovascular eye diseases. Nature Communications 16, 4791 (2025). https://doi.org/10.1038/s41467-025-59929-2