AI-Integrated Imaging Reveals Retinal Cellular Structures with Precision and Speed
A new cost-efficient and open-source technology could revolutionize the diagnosis and treatment of eye and brain diseases.
Biomedical engineers at Duke University have developed a novel imaging system that harnesses the power of artificial intelligence to visualize individual retinal cells better than more expensive technologies. This advance could greatly enhance modern medicine’s diagnostic and monitoring capabilities for a range of diseases.
The research appeared in Science Advances.
The retina, a thin layer of light-sensitive cells at the back of the eye, plays a vital role in converting and transmitting visual information to the brain. As an extension of the central nervous system, it offers researchers a unique, non-invasive means to visualize neurons at the cellular level.
"These retinal cells serve as surrogates for studying diseases of the brain, such as Alzheimer’s or multiple sclerosis. Precise imaging is crucial for early detection. With clearer images, we can identify diseases earlier and evaluate experimental therapies more effectively than other brain imaging methods", says Sina Farsiu, Anderson-Rupp Professor of Biomedical Engineering and Ophthalmology at Duke University.
The most commonly used method for imaging retinal cells is adaptive optics scanning light ophthalmoscopy (AOSLO). Traditional AOSLO images are formed only from directly reflected light off the retina. However, these images often contain misleading artifacts, prompting modern AOSLO systems to incorporate non-confocal information from indirectly reflected light to resolve retinal cells.
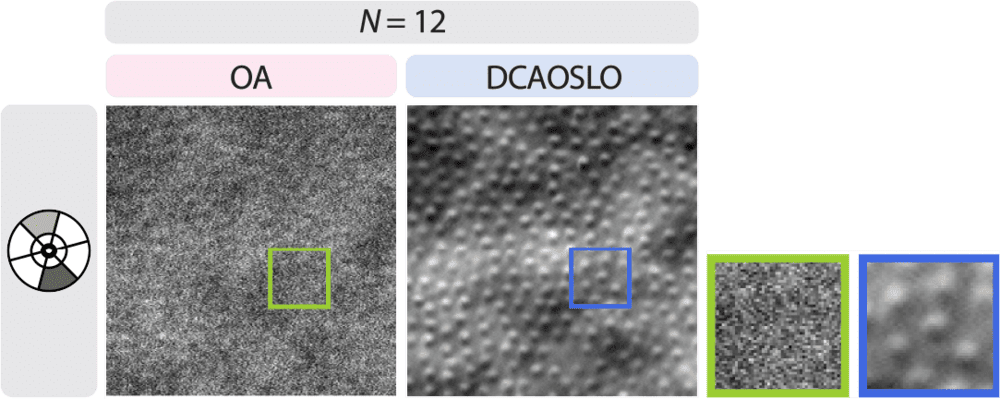
Comparison of photoreceptor imaging using a state-of-the-art AOSLO setup (OA, left) versus DCAOSLO at similar imaging times. Image: Science Advances
“Non-confocal AOSLO methods typically employ only two sensors to capture scattered light. While this provides insights from one angle, it falls short in fully capturing a retinal structure’s shape and health,” Farsiu explained. “For example, horizontally oriented vessels might be correctly resolved while vertically oriented ones are missed.”
Efforts to address this challenge have relied on additional sensors, which provide detailed information about microstructures but require expensive and complex equipment. Alternatively, researchers can adjust a sensor’s position after each scan, but this method drastically increases imaging time, impacting patient comfort and safety.

Each image produced by a pair of non-confocal AOSLO channels reveals information that may be obscured in images captured by differently oriented channels. The red and blue arrows highlight microvasculature that appears or is missed in two non-confocal AOSLO images of the retina. Image: Science Advances
To overcome these limitations, Farsiu and his team developed a novel approach called Deep-Compressed AOSLO (DCAOSLO). This method uses compressed sensing, a signal processing technique that requires only a few projections of the imaged tissue to rapidly reconstruct images. By employing an array of tiny mirrors tilted via software to collect retinal light reflections, DCAOSLO captures essential features without the need for the time-consuming sensor-by-sensor scanning used in standard AOSLO. The data is then processed by an AI algorithm to create images as if generated by multiple sensors.
“DCAOSLO can simultaneously capture scattered light from 12 sensor positions with only two sensors, while significantly reducing cost and imaging time compared to conventional AOSLO,” said Jongwan Park, a PhD student in Farsiu’s lab and the paper’s first author. “Our hardware modifications are minimal, and researchers can easily incorporate them into existing AOSLO setups.
To demonstrate DCAOSLO’s capabilities, the team used it to image various retinal structures in both healthy and diseased subjects. The system not only improved images of rods, cones, and blood vessel cells but also achieved imaging speeds nearly 100 times faster than conventional AOSLO setups.
“Single-cell retinal imaging systems, including AOSLO, will only see widespread clinical adoption if imaging can be performed rapidly, accurately, and cost-effectively,” said Farsiu. “DCAOSLO is a practical diagnostic tool with the potential to transform the management of neurological, cardiovascular, diabetic, and retinal diseases.”
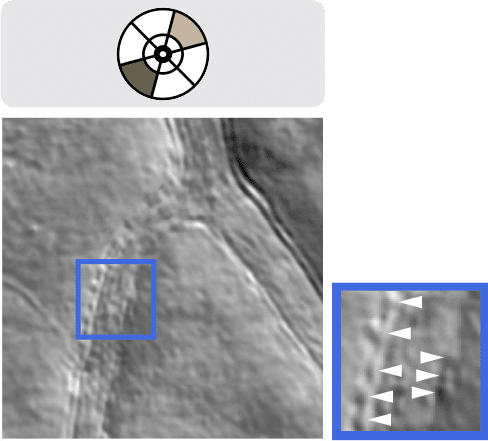
DCAOSLO image enabling visualization of detailed vessel wall structures, including endothelial cells and surrounding mural cells (e.g., pericytes, white arrowheads), a potential early biomarker for diabetic retinopathy and glaucoma. Image: Science Advances
In summary, according to the research team, DCAOSLO is a rapid nonconfocal AOSLO imaging technique that compressively samples the 2D nonconfocal light intensity and reconstructs nonconfocal channel images using the state-of-the-art CNN-based algorithm that does not require training data. They have demonstrated DCAOSLO’s superior imaging performance over conventional OA imaging with markedly faster acquisition speed in both healthy control participants and patients with ocular diseases in vivo. DCAOSLO’s ability to provide fast and accurate visualization of various retinal cellular structures has the potential to cause a paradigm shift in the way ocular disease screening is performed, providing practical diagnostic tools and methodologies that could revolutionize the management of neurological and retinal diseases.
Jongwan Park et al., Deep compressed multichannel adaptive optics scanning light ophthalmoscope.Sci. Adv.11,eadr5912(2025).DOI:10.1126/sciadv.adr5912
Source: Duke University / Pratt School of Engineering