Uveal Melanoma: Patient-Derived Organoid Models Offer Hope for Improved Treatments
Mayo Clinic researchers have developed patient-derived organoid models to study uveal melanoma. Their goal is to use these models to better understand how this disease works and develop treatments for unmet patient needs.
In 50% of patients, uveal melanoma metastasizes, spreading to other parts of the body, leading to a poor prognosis and average survival of less than two years. Unfortunately, current treatments for this condition often have limited effectiveness, leaving patients and their doctors with few options.
“Improved outcomes for patients”
Organoids are 3D models grown from patient tissue that accurately reflect a patient's unique genetic and biological characteristics, also known as "avatars." When derived from a patient's cancer tumor, an organoid will behave and respond to treatments outside the body in vitro just like the original tumor would in vivo.
"The hope is that these patient-derived organoid models better represent human cancer in the laboratory," says Lauren Dalvin, M.D., a Mayo Clinic Comprehensive Cancer Center ocular oncologist and surgeon-scientist who is one of the lead researchers. "Using these models as a foundation for drug testing will facilitate new treatment discoveries with higher success rates in clinical trials, ultimately translating to improved outcomes for patients with uveal melanoma."
In the past, the lack of human disease models representing the entire spectrum of uveal melanoma has created a bottleneck, limiting the ability of scientists to identify effective targets for treatment and prevention. Most laboratory studies have drawn from the same set of commercially available cell lines, which are not representative of the disease and often differ in important ways from the original tumors.
New, uveal melanoma patient-derived organoid biobank
To blast through this bottleneck, a study team led by Dr. Dalvin, in collaboration with Martin Fernandez-Zapico, M.D., a cancer biologist with Mayo Clinic Comprehensive Cancer Center, decided to develop a new, uveal melanoma patient-derived organoid biobank. Their goal is to create a research resource representing the real-world variability of this cancer.
In a paper published in Investigative Ophthalmology & Visual Science, they described the initial development of this biobank. The researchers successfully created organoids derived from Mayo Clinic ocular oncology patients. Uveal melanoma patients requiring enucleation from 2019 to 2024 donated tumor tissue for generation of patient-derived organoids (PDOs). PDOs were cultured in Cultrex and compared to donor primary tumor using exome sequencing, RNA sequencing, and immunohistochemistry. The ability of PDOs to maintain the transformed phenotype was evaluated in an orthotopic xenograft model and monitored with fundus imaging. ATAC sequencing and drug response assays were done in a subset of PDOs to explore the feasibility of their use for mechanistic and translational studies. PDOs were successfully established in 40 of 44 cases (91%).
Their study determined that these organoid models:
- Could be generated, retained their stability through many uses and were a renewable living resource capable of being regenerated at need.
- Retained the clinically relevant features of the original tumors, clustered into appropriate molecular groups based on validated prognostic markers and resembled human disease when compared to in vivo animal models.
- Served as suitable human models for drug screening.
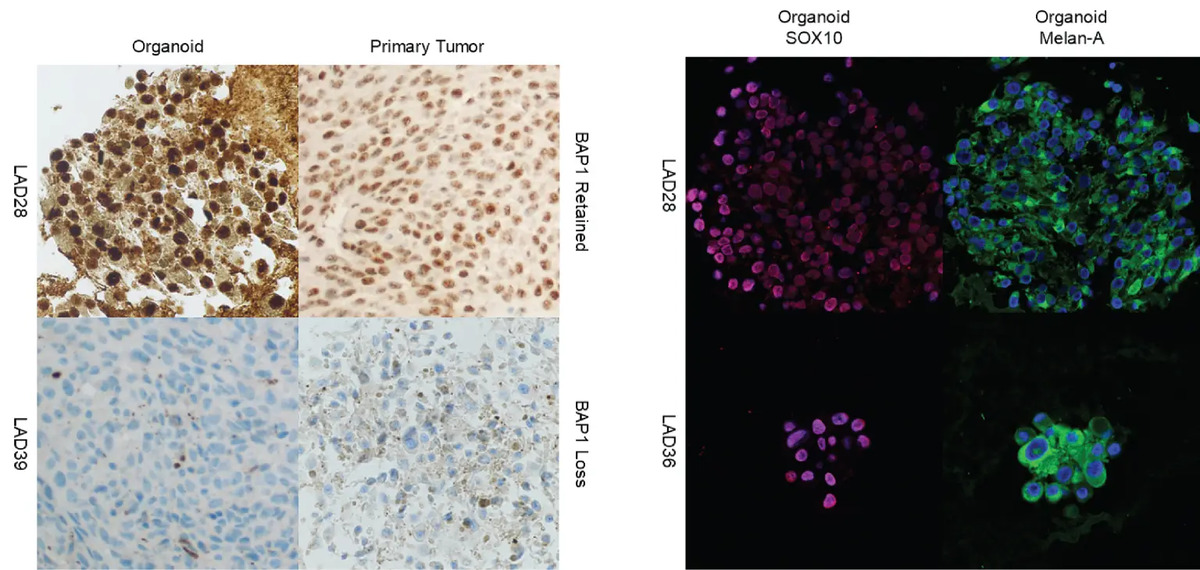
Images at the left show two uveal melanoma patient-derived organoids side-by-side with corresponding original samples from their primary tumors. Under a microscope, the lab-grown cells look very similar to the original tumor. Importantly, a key feature of the tumor, related to a protein called BAP1, is also consistent.
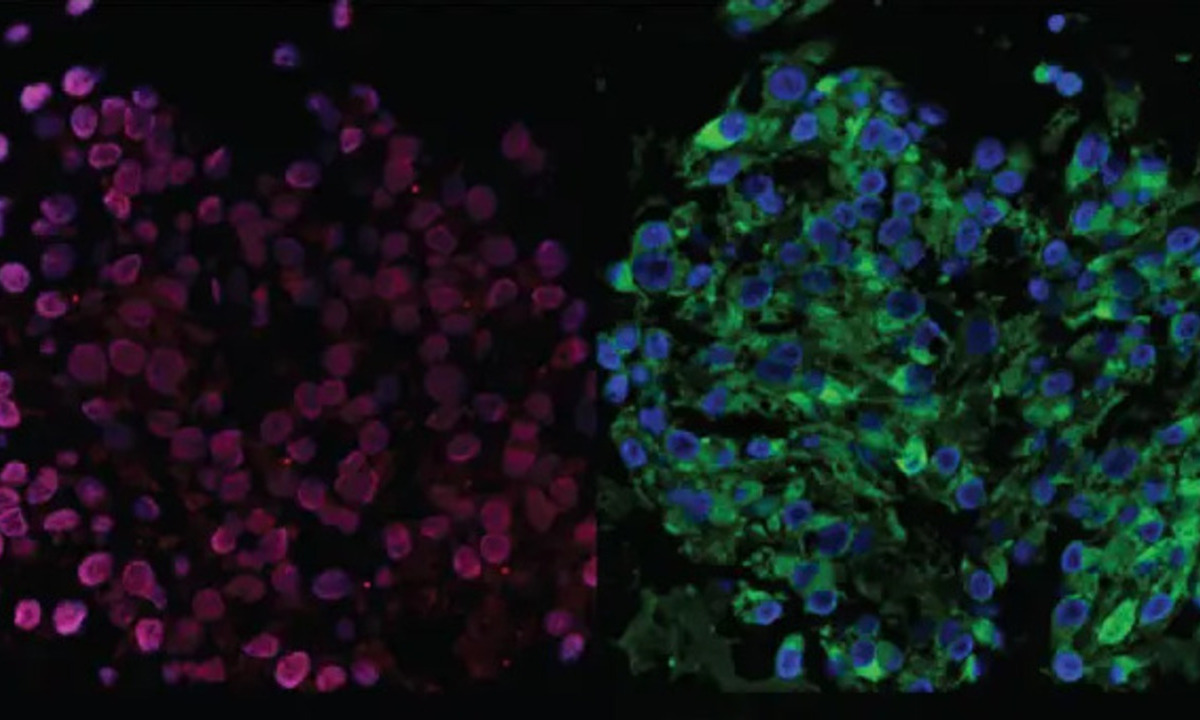
Images at right show two organoids examined using immunofluorescence to highlight different parts of the cells. The presence of these stains in specific locations within the cells confirms that the lab-grown organoids are derived from melanocytes, which are the cells that produce pigment in the eye.
Recognizing the immense value of this organoid biobank, the investigators have already begun expanding it to include other research centers. Their goal is to create a resource capable of representing the global epigenomic variability of uveal melanoma. In the future, they hope this biobank will serve as a comprehensive platform for drug screening and other types of lab research on uveal melanoma. This collaborative effort will accelerate research and pave the way for improved treatments and outcomes for patients with this disease.
Source: Mayo Clinic
Reference:
Lauren A. Dalvin et al, Novel Uveal Melanoma Patient-Derived Organoid Models Recapitulate Human Disease to Support Translational Research. Invest Ophthalmol Vis Sci. 2024 Nov 4;65(13):60. doi: 10.1167/iovs.65.13.60.